The Telomere Syndrome Does Not Cause Aging as Manifested in Telomere-Mediated Organ Failure and Cancer
Rationale:Seventy-six millions of the baby boomers approach late adulthood, requiring medical treatment and ~100,000 individuals of the US population are affected by telomere syndrome, an autosomal dominant disorder, marked by grey hair as early as the age on nine. Telomere shortening is strongly implicated in aging. As cells replicate, telomeres shorten and limit the number of times a cell can divide, affecting degeneration (Armanios “Interview”). Unlike aging, the telomere syndrome expresses fundamental heterogenous manifestations between diverse people (“Telomere Dysfunction” 5099). Research into the heterogenous nature of telomere syndromes will provide insight into its relationship with aging.
Objectives: I sought to identify the appropriate terminology to be used when diagnosing and treating patients affected by telomere syndromes to direct suitable patient-physician interactions.
Measurements and main results: Oncologists name the telomere syndrome as age-associated because some cases of telomere shortening reported having hair graying, organ failure, and cancer which usually appear in individuals older than 60 years of age. However, scientists’ works hint that telomere syndrome should not be defined as an aging disease and telomere-syndrome-affected patients described as aged. This suggestion comes because, opposite to age-related disorders, which are primarily driven by gradual telomere length and neuropsychological decline over many years, telomere-mediated organ failure is sudden and primarily driven by congenital, critically short telomere length and environmental risk factors, like smoking and psychological stressors.
Conclusions: Our data indicate that 1) the short-telomere patients may not show the difference in onset, sex and socioeconomic status as well as the primary physical, mental, or psychological signs of aging and 2) environmental stressors, like smoking and psychological state are the primary factors lowering the threshold of telomere-syndrome-induced organ failure and cancer, regardless of age. Aging is a different process from telomere-mediated degeneration.
Telomeres are tandem repeat of TTAGGG extending several kilobases at the end of chromosomes to prevent their fusion and allow their transmission along generations. Telomerase synthesizes the telomeres, which are made up of two components. Mutations in telomerase’s hTERT and hTR and short telomeres underlie the telomere-syndrome-mediated idiopathic pulmonary fibrosis (IPF) and emphysema, progressive degenerative lung diseases, which cause the highest morbidity, 90%, and are most common among telomere-mediated disorders. While age-related lung disease may be developed over “many years” through gradual degeneration of the organs, telomere-syndrome-mediated degenerative disease has a relatively quick manifestation, primarily driven by congenital, “critically short” telomere length and environmental stressors, like smoking as well as feeling threatened and on-edge, affecting all age groups indiscriminately (Blackburn and Epel “Interview,” Armanios and Blackburn 693-704).
Here, I argue that clinicians fail to acknowledge several points when describing the telomere syndrome as age-related or defining the telomere-syndrome-affected patients as aged. They fail to recognize that 1) the differences in onset, sex and socioeconomic status as well as the primary physical, mental, and psychological signs of aging are, more often than not, absent in individuals with telomere syndromes and 2) telomere syndromes do not occur mainly based on the genetics or the person’s age group, like aging, but primarily due to environmental stressors acting on congenital, critically short telomeres.
Absence of Physical, Mental and Psychological Signs of Aging as well as Link to Socioeconomic Status in Persons with Telomere Syndromes Shows that Telomere Syndrome is not an Aging Disease
Labeling telomere-syndrome patients as aged undermines the fact that, unlike aging, telomere syndrome affects women more than men, does not differentiate based on the socioeconomic status, and patients lack the neuropsychological signs of aging. According to Dr. Laura Berk, aging includes physical limitations that manifest in daily activities, like basic self-care. Also, the brain weight declines more rapidly, vision impairs, hearing declines, senses of taste and smell reduce, and cardiovascular effects, like atherosclerosis are significant signs for men more than women. These aging phenotypes are variable with the socioeconomic status (612-14). In contrast, telomere-syndrome-affected patients do not show a decline in physical abilities. Furthermore, in contrast to aging, telomere-syndrome-mediated degeneration affects women more than men. Likewise, remarkably, unlike aging, the severity of telomere syndrome is not directly linked to the person’s socioeconomic status (Armanios 996-1002 and “Interview”).
From a social perspective, Berk describes the disengagement and activity social theories of withdrawal between older adults and society, happening in anticipation of death as hallmarks of aging (614). However, telomere-syndrome-affected patients feel arousals and the excitement of youth and teenage years as well as strongly seek family ties and social connections for reinforcement (Armanios “Interview”). The neuropsychological phenotypes and differences in sex, defining aging are not manifest in telomere-syndrome-mediated degeneration, explaining why it may not be age related.
Contrary to Aging, which is primarily driven Genetics and the Person’s Age Group, Telomere-syndrome-Mediated Organ Failure is Primarily Driven by Environmental Stressors and is an Equal Chance for Old and Young Persons, therefore it is not an Aging Disease
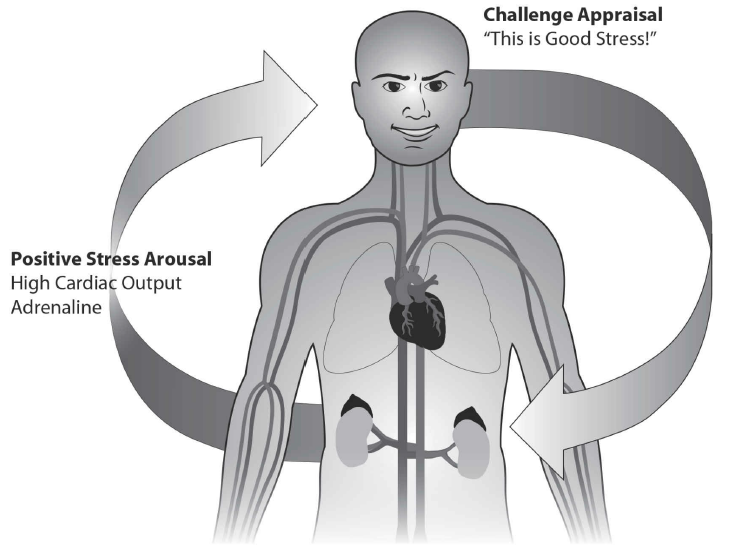
Not only are aging phenotypes absent from telomere-syndrome patients, but also young and old, telomere-syndrome persons are equally susceptible to have the effects of the disease. Dr. Blackburn and Dr. Epel maintain that feeling threatened and on-edge shortens telomeres in immune system cells “over many years,” constricts blood vessels, decreases the cardiac output, and withdraws the vagus nerve’s activity, which supports the heart and muscles. As a result, the immune system weakens, causing severe infections and cells starve for nutrients and oxygen, accelerating degeneration (6-11). The opposite holds true (see fig. 1). The three following types of failure at the organ and cellular levels are instances where telomere-syndrome differs from aging. As a result, telomere syndrome is not an aging disease.
Lung Disease (IPF and emphysema).
Contrary to age-related IPF and emphysema, which are genetically- and age-based, telomere-syndrome-mediated degeneration is genetically determined by telomerase mutation present at birth as well as an acquired (e.g. cigarette smoke-induced) damage. In a meta-analysis of 14 studies including a cohort of 934 chronic obstructive pulmonary disease (COPD) cases with 15,846 controls defined according to the Global Lungs Initiative (GLI) criteria and spirometric parameters of 12,595 individuals, Albrecht et al. found that telomere length in the circulating leukocytes “contribute to the pathogenesis of COPD” and emphysema, with smoking acting as a primary driver (983).
Interestingly, when Albrecht et al. conducted an age-sensitivity study to determine the contribution of the individuals’ age by ruling out subjects >60 years of age, they found no difference in data. They also found that women were more susceptible than men (983-992). In other words, these studies indicated that unlike the age-related lung disease, the telomere-syndrome-mediated shows a quick onset, primarily driven by environmental stressors regardless of age and occurs in women more than men.
Bone marrow (stem cell) failure.
Preliminary forms of the telomere syndrome, like hair graying do not progress to greater levels, like immunoscence in infants without environmental stressors. In the interview with Dr. Mary Armanios, she notes that a college student comes to the hematology clinic at Johns Hopkins for bone marrow failure who has had grey hair since the age of nine. Despite having congenital, critically short telomere length, the boy did not develop bone marrow failure until he faced college stress (“Interview” and Blackburn and Epel 14). Therefore, telomere syndrome is not simply associated with age because the boy did not develop immunoscence solely based on his age group. Bone marrow failure is known to only occur in people older than 60 years of age (“Telomere Dysfunction” 5099). However, stress from college is likely the primary factor promoting the progressive degenerative manifestation of telomerase mutations, which the boy possessed at birth.
Cancer.
The interaction between genetics and the environment, namely “elderlyspeak” if “people mutely accept the attitudes behind them,” can cause cancer (Pietrzak 2016). Elderlyspeak is “the sweetly belittling form of address [to] older people” (Leland and Kolata). So, designating telomere syndromes as age-associated and addressing such patients, using elderlyspeak may be the factor causing cancer than the other way around.
For example, Ms. Koenig, working for Y-ME National Breast Cancer Organization and receiving 40,000 calls a year, reports many telomere-syndrome patients being worried that elderlyspeak caused their cancer (Kolata). Dr. Hannun and Dr. Newcomb established a link between ceramide, a chemical produced as a result of several stress stimuli, and cancer metastasis (692-94). From these discussions, it is clear that cancer does not develop in short-telomere patients merely because of aging. Environmental stressors, including physicians and family elderlyspeak could be the primary factor leading short telomere patients to develop cancer. Therefore, telomere syndrome is not an aging disease.
Addressing counterarguments.
When clinicians see young patients with telomere syndrome have grey hair, the disease is labeled as an aging disease because white hair is not usually seen in young people. This is a misconception that people with white hair are old, neglecting the differences in sex and socioeconomic status as well as the neuropsychological and etiological differences between telomere syndrome and aging.
While some may argue that cancer affects infants without prior exposure to stress, Dr. Blackburn and Dr. Epel maintain that nature and nurture simultaneously play a part. For example, while the infant brain may not have reached the age to conceive threat, the mother’s stress during conception may have harmed the baby’s cells indirectly (14). Dr. Hannun and Dr. Newcomb’s work suggests that the elevated levels of ceramide in the mother’s body may elicit tumorigenesis in the infant’s body (692-94).
Discussion and Conclusion
Labeling all patients with grey hair due to short telomere as aged will form a stereotype for those group of people, which is not accurate and does not apply to them. This situation may result in misinformed decisions regarding etiology, diagnosis, and treatment of any of the manifestations of the telomere syndrome. Furthermore, categorizing the telomere syndrome as age-related may put more emphasis for screening and genetic testing of older people and men than young people and women who may be equally and more susceptible, respectively. This discussion will spur research about biomarkers for stress-mediated diseases, including cancer and whether elderlyspeak is directly linked to cancer in telomere syndrome patients. It may also play a role in elucidating the etiology behind the large impact that adverse childhood events (ACEs) have on the health of individuals as they age (Niel et al. 549).
Works Cited
Albrecht, E, et al. “Telomere length in circulating leukocytes is associated with lung function and disease.” European Respiratory Journal 2014, vol. 43, pp. 983-992.
Alder JK, et al. “Telomere dysfunction causes alveolar stem cell failure.” Proceedings of the National Academy of Sciences USA vol. 112, no. 16, 11 March 2015, pp. 5099-5104.
Alder, JK, et al. “Telomere length is a determinant of emphysema susceptibility.” American Journal of Respiratory and Critical Care Medicine vol. 184, no. 10, 15 Oct. 2011, pp. 904-912.
Armanios, M. Interview by Roger Bingham. Interview with Mary Armanios, 14 June 2011. Web.
Armanios, M. “Telomeres and age-related disease: how telomere biology informs clinical paradigms.” Journal of Clinical Investigation vol. 123, no. 3, March 2013, pp. 123:996-1002.
Armanios, M, and Blackburn, EH. “The telomere syndromes.” Nature Reviews Genetics vol.13, no. 10, 1 Oct. 2012, pp. 693-704.
Berk, L. Development through the Lifespan. New Jersey: Pearson, 2014. Print.
Blackburn, E and Epel, E. The Telomere Effect. New York: Grand Central Publishing, 2017. Print.
Blackburn, E and Epel, E. Interview by Annahad O’Connor. Dr. Elizabeth Blackburn & Dr. Elissa Epel, 10 Jan. 2017, www.youtube.com/watch?time_continue=6&v=Nki_hHblDP4.
Hannun YA and Newcomb B. “New twist to the emerging functions of ceramides in cancer: novel role for platelet acid sphingomyelinase in cancer metastasis.” EMBO Molecular Medicine vol. 7, no. 6, 9 April 2015, pp. 692-94.
Kolata, G. “Is there a link between stress and cancer.” Nytimes.com, 29 Nov 2005. Web.
Leland, John. “In ‘Sweetie’ and ‘Dear,’ a Hurt for the Elderly.” Nytimes.com, 6 Oct 2008. Web.
Niel VC, Pachter LM, Wade R, Felitti VJ, and Stein MT. “Adverse Events in Children: Predictors of Adult Physical and Mental Conditions.” Journal of Developmental and Behavioral Psychology vol. 35, no. 8, Oct. 2014, pp. 549-551.
Pietrzak, RH, Zhu, Y, Slade, MD, Qi, Q, Kryztal, JH, and Levy, BR. “Association between negative age stereotypes and accelerated cellular aging: Evidence from two cohorts of older adults.” Journal of the American Geriatrics Society vol. 64, no. 11, Nov. 2016, pp. e228-e230.
Reflection
This essay helped me be a more critical reader of scientific texts, summarize texts, combine evidence, and locate an argument within the conversation. Then I could determine my own stance and organize my essay in a way that best supports my thesis. Printing out, reviewing, and marking earlier drafts helped me improve significantly and shape my argument. Asking different people to look at my writing and discussing with them, helped refine my ideas. I used They say| I say templates to show that I am engaging in conversation with other writers and why it matters: first sentence in the essay, where I used, “___used to think___. But recently [or within the past few decades] ___ suggests that ____.” I also use metacommentary, “in other words…” I also addressed counterarguments.
I used They say| I say templates to show that I am engaging in conversation with other writers and why it matters: first sentence in the essay, where I used, “___used to think___. But recently [or within the past few decades] ___ suggests that ____.” I also use metacommentary, “in other words…” I also addressed counterarguments.
The hardest part was organizing the essay, but following the “old/new contract” helped me. It took me a while to figure out the sections and subsections and to trim the essay within the word limit to focus and be concise. The easiest parts were finding papers and researching databases.